Abstract
Introduction: Accurate assessment of the risk of non-relapse mortality (NRM) is important for making the shared decision about treatment with allogeneic hematopoietic cell transplantation (allo-HCT). We have shown that the pre-transplantation plasma level of suppression of tumorigenicity 2 (ST2)-a protein that is released to the bloodstream upon inflammation, cellular stress and endothelial damage-was associated with NRM after myeloablative allo-HCT [Gjærde et al., ASH Annual Meeting 2020, abstract #1524]. In an expanded cohort of both myeloablative- and non-myeloablative conditioned patients, we aimed to validate the value of pre-transplant ST2 in predicting 1-year NRM after allo-HCT.
Methods: Pre-transplantation plasma ST2 levels were measured by enzyme-linked immunosorbent assays in 374 adult patients who underwent allo-HCT at Rigshospitalet between July 2015 and December 2019 (Table 1), using stored plasma samples collected at a median (Q1, Q3) of 23 (21, 24) days before allo-HCT. All patients were followed-up for at least 1 year after transplant. NRM was defined as all deaths in relapse-free patients. Given our sample size and outcome proportion, we could include four parameters in a logistic regression model of 1-year NRM to avoid severe overfitting [Riley et al., BMJ, 2020]. Based on our current clinical risk assessment practice, we included age (linear), comorbidity index (HCT-CI [Sorror et al., Blood, 2005], linear) and conditioning intensity (myeloablative vs. non-myeloablative) in a base model, to which we added the pre-transplantation ST2 level (linear) and assessed its incremental prognostic value [Steyerberg et al., Epidemiology, 2019]. The internal validity of the full model was estimated by bootstrapping [Steyerberg et al., J Clin Epidemiol, 2001].
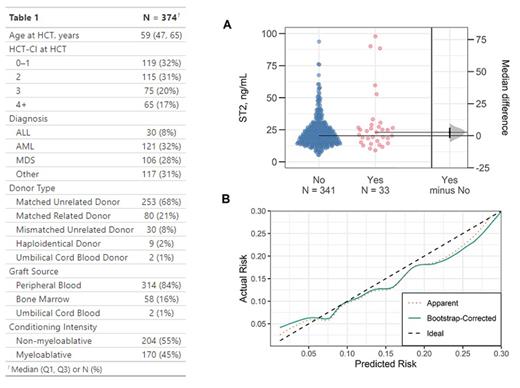
Results: The median (Q1, Q3) pre-transplantation plasma ST2 level was 20.4 (15.2, 27.2) ng/mL. NRM at 1-year was 9% (N = 33). The main causes of NRM were organ failure (39%), infection (23%) and acute graft-versus-host disease (21%). Relapse risk at 1-year was 18%. The patients who constituted the 33 cases of 1-year NRM had a 2.7 ng/mL higher median pre-transplantation ST2 level than the remaining 341 patients (95% bootstrap confidence interval [CI] of the difference: -1.9, 6.2 ng/mL, Figure Panel A). In the full logistic regression model-including age, HCT-CI, conditioning intensity and ST2-ST2 was associated with 1-year NRM with an odds ratio of 1.32 (CI: 1.05, 1.65) per 10 ng/mL increase. Adding ST2 to the base model increased the model likelihood ratio χ 2 from 12.1 to 17.3 (p = 0.02), i.e. ST2 added a fraction of 30% (12.1/17.3) of new predictive information to age, HCT-CI and conditioning intensity. However, the ability of the full model to discriminate cases of NRM at 1-year remained poor with minimal improvement after adding ST2 (AUC up to 0.675 from 0.674 in the base model). The bootstrap-corrected AUC (the expected AUC of the full model used in a new population) was 0.63. Moreover, bootstrap-corrected estimates of predicted vs. observed risk revealed slight model miscalibration: lower predicted risks were generally underestimated, while higher predicted risks were overestimated (Figure Panel B).
Conclusion: Pre-transplantation plasma levels of ST2 was a prognostic biomarker of 1-year NRM after allo-HCT, adding new predictive information to age, HCT-CI and conditioning intensity. However, internal validation of the full ST2-based prediction model revealed poor overall performance, precluding further validation and use of the model in clinical practice. When identifying prognostic biomarkers, investigation of overall predictive performance (in addition to already known prognostic factors) is needed before clinical usefulness can be evaluated.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal